OpenSourcePV
Transparent Safety Monitoring
About
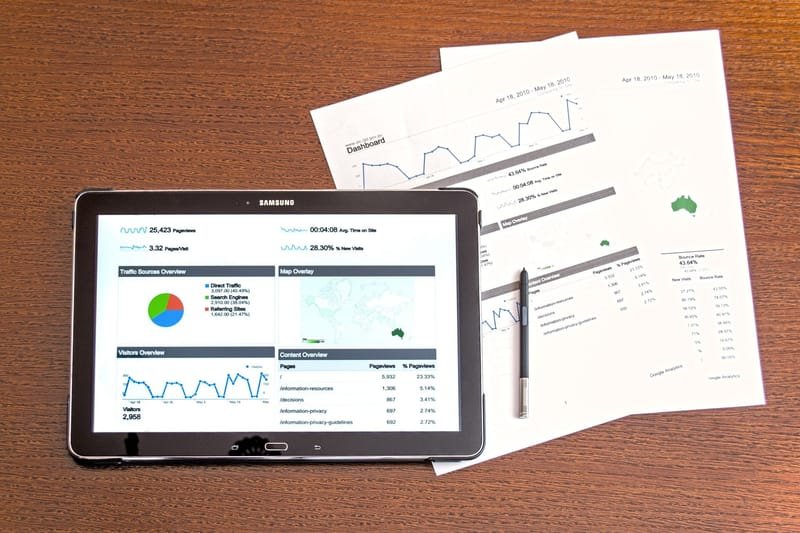
To meet their regulatory obligations, pharma companies need to monitor the safety of their products. When they start to have a strong pipeline and multiple products on the market, the amount of data to be reviewed is soon to large to be reviewed manually. To benefit from quantitative analysis, they currently have two options: either they use an expensive suite of tools or they try to build something on their own.
With OpenSourcePV, we accompany their journey in quantitative signal detection allowing them to benefit from pre-built modules and building something customized to their specific needs and data sources. Our mantra is transparency, so whatever is built for them will be owned by them with the ability to modify it to adapt to their future needs. The existing modules are the following ones 'VAERS Analytics', 'FAERS Analytics', 'StandAlone Signal Detection' on their own spontaneous report data and 'Competitive Intelligence' to compare safety profile to existing competitors on the market.
SOLUTIONS & Services

Software as a Service (SaaS)
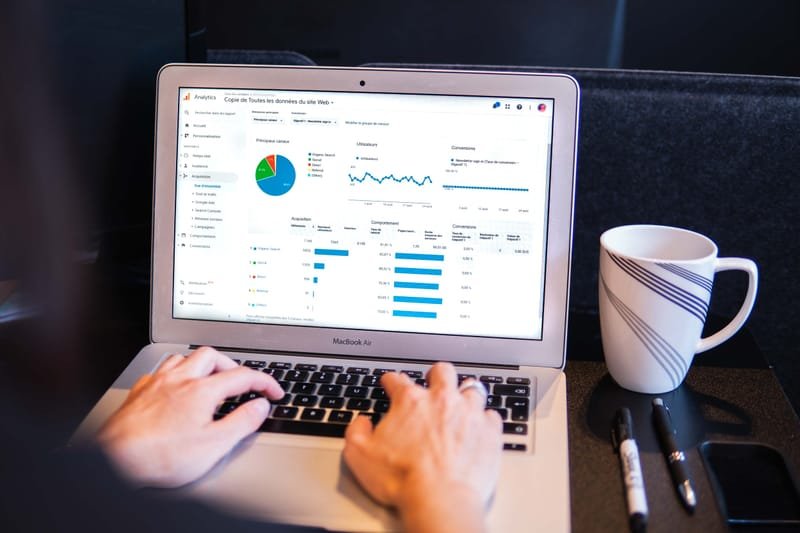
Three SaaS are available to the customers 'VAERS Analytics', 'FAERS Analytics' and 'Stand Alone Signal Detection' so that they can get safety insight from the CDC/FDA regulatory database and their own spontaneous report data.
Learn More
Consulting
Methodological support in quantitative signal detection, observed vs expected, time-to-onset signal detection, implementation of open source technology in the field of pharmacovigilance
Learn More
Public Health Initiatives
Build a signal detection and management tool for the pharmacovigilance community that can be used during crisis times like epidemics for evaluating safety data in parallel from the market authorization holder and the regulatory authorities.
Learn More
Competitive intelligence
You are not first-to-market and would like to know how the safety profile of your product differentiates from the competitor.
Learn More